Soluble alkaline-earth metal hydrides
Calcium hydride (CaH2) is a salt that is fully insoluble in organic solvents. With the right ligand it can be brought into solution in aromatic solvents and becomes highly reactive. Even more reactive are the recently discovered heavier Sr and Ba hydride clusters.
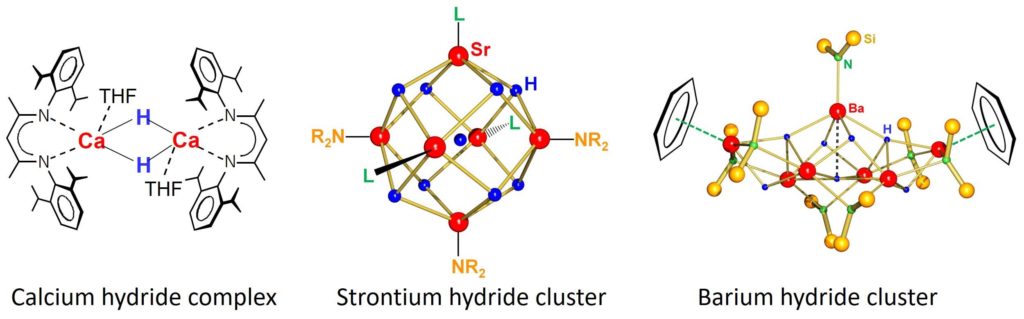
Angew. Chem., Int. Ed. 2006, 45, 3474
Rational Design of a Well-Defined Soluble Calcium Hydride Complex
DOI: Angew. Chem. Int. Ed.10.1002/anie.200601013
Angew. Chem., Int. Ed. 2017, 56, 11880
A Simple Route to Calcium and Strontium Hydride Clusters
DOI: Angew. Chem. Int. Ed.10.1002/anie.201706786
Angew. Chem., Int. Ed. 2017, 56, 16654
Simple Access to the Heaviest Alkaline Earth Metal Hydride: A Strongly Reducing Hydrocarbon-Soluble Barium Hydride Cluster
DOI: Angew. Chem. Int. Ed.10.1002/anie.201709771