Hydrogenation catalysis
In 2008 we discovered first transition metal-free catalysts for alkene hydrogenation. The last decade has seen continuous improvements in catalyst design and performance. Even simple amide complexes can be catalytically active for imines and alkenes. Bulky Ba amide complexes catalyze (slow) benzene hydrogenation!
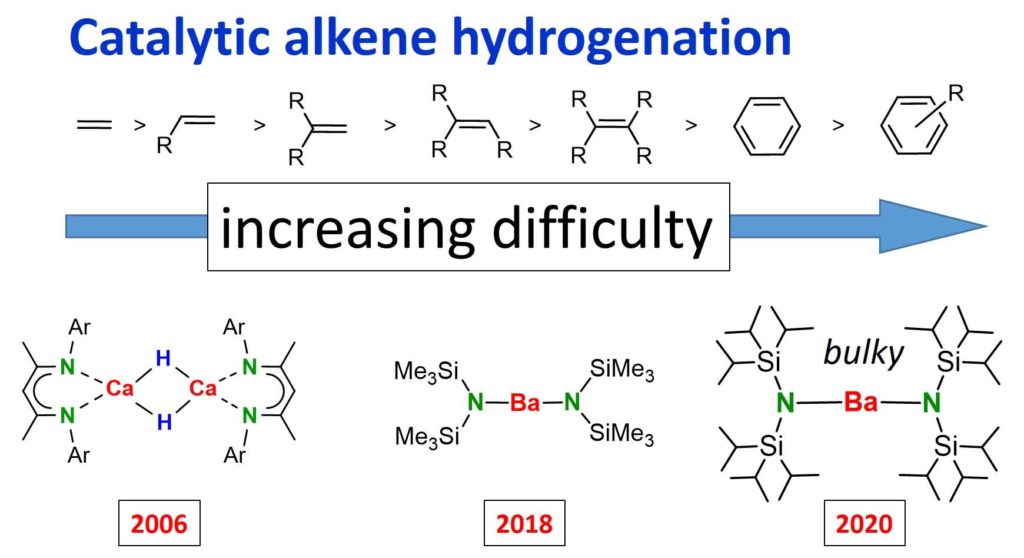
Nature Catalysis, 2018, 1, 40-47
Imine Hydrogenation with Simple Alkaline Earth Metal Catalysts
DOI: 10.1038/s41929-017-0006-0
Angew. Chem., Int. Ed. 2018, 57, 15177
Simple Alkaline-Earth Metal Catalysts for Effective Alkene Hydrogenation
DOI: Angew. Chem. Int. Ed.10.1002/anie.201810026
Angew. Chem., Int. Ed. 2020, 59, 9102
Highly Active Superbulky Alkaline Earth Metal Amide Catalysts for Hydrogenation of
Challenging Alkenes and Aromatic Rings
DOI: Angew. Chem. Int. Ed.:10.1002/anie.202001160