Bimetallic Catalysis
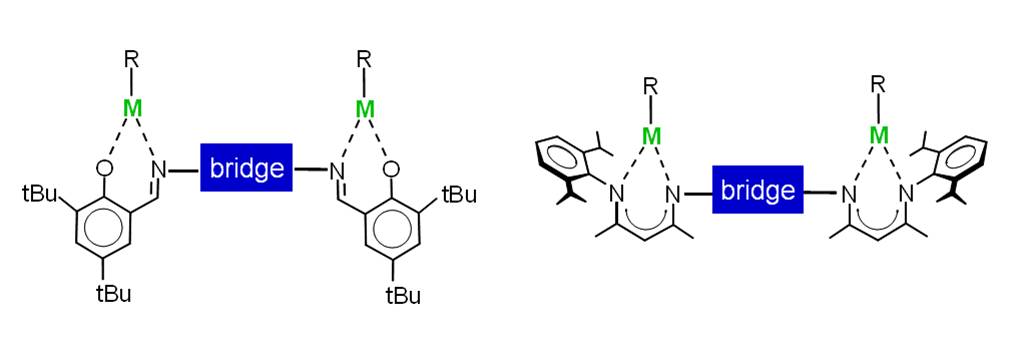
Most enzymes are multimetallic catalysts that cooperate efficiently in order to accomplish their specific tasks. We have developed a variety of bimetallic catalysts that have been tested in the copolymerization of cyclohexene oxide and CO2. The dimeric Zn catalyst for this reaction suffers from a dimer-monomer equilibrium. As the monomer is inactive, polymerizations are run at high concentration (this maximizes the dimer content). High concentrations, however, limit the monomer conversion.
We developed a convenient synthetic route to bimetallic Zn catalysts that are also active under dilute reaction conditions and give complete monomer conversion.
(Piesik, Range, Harder Organometallics 2008)